Clinical Research Experts Work with Your Documents for Higher Translation Accuracy
Our team comprises of field specialists dedicated to your subject, ensuring the utmost precision in clinical trial translations. Each translator holds a Ph.D. or Master's degree in the relevant subject, coupled with extensive years of translation experience.

PhD
in Medicinal Chemistry, The Central University of Venezuela
24+ Years of Experience


MS
Microbiology, Southern Illinois University, USA
20+ Years of Experience


PhD
in Medical Sciences, Nagoya University, Japan
13+ Years of Experience


Doctor
Dental Medicine, Public Health, Universidade de Lins, Brazil
12+ Years of Experience


Doctor
of Pharmacy (Pharm.D.), Pharmacy and Biochemistry
10+ Years of Experience

200+
Languages
125+
Countries
3k+
Language Experts
200k+
Clients Worldwide

Terminology Databases Ensure Accurate Translation of Complicated Medical Terms
Terminology databases play a crucial role in ensuring the precise translation of complex medical terms. Alongside MedDRA and our proprietary terminology tool - GlossaryX360, we cover over 100,000 medical terms. This comprehensive coverage not only facilitates accurate translations of industry-specific terms but also contributes to faster turnaround times.
Clinical Trial Documents We Specialize in
- Clinical Trial Protocols
- Informed Consent Forms (ICFs)
- Patient Information Leaflets (PILs)
- Case Report Forms (CRFs)
- Regulatory Submissions
- Clinical Trial Reports
- Labeling and Packaging Materials
- Quality of Life Questionnaires
- Safety and Adverse Event Reports
- Clinical Outcomes Assessments (COA) & (eCOA)

Regulatory Compliance for faster approvals
Our translations are made compliant to global regulatory requirements based on your request. We can also format your documents to ensure they are compatible with the relevant Clinical Trial Information System (CTI).
- The European Union
- The European Medicines Agency (EMA)
- World Health Organization
- Food and Drug Administration (FDA)
- Countries national regulations
- International Conference on Harmonization (ICH)
- Local Institutional Review Boards (IRBs)
Clinical Trial Translations
You Can Trust
We've designed our localization experience with certified processes and efficient technology, ensuring a seamless delivery of consistent quality that enhances client satisfaction.

Specialized Subject-
Area Matching

Quality-
Driven Technology

24x7 Dedicated
Localization Manager

ROI-Driven
Localization Solutions

In-House Expertise

ISO-Certified Processes
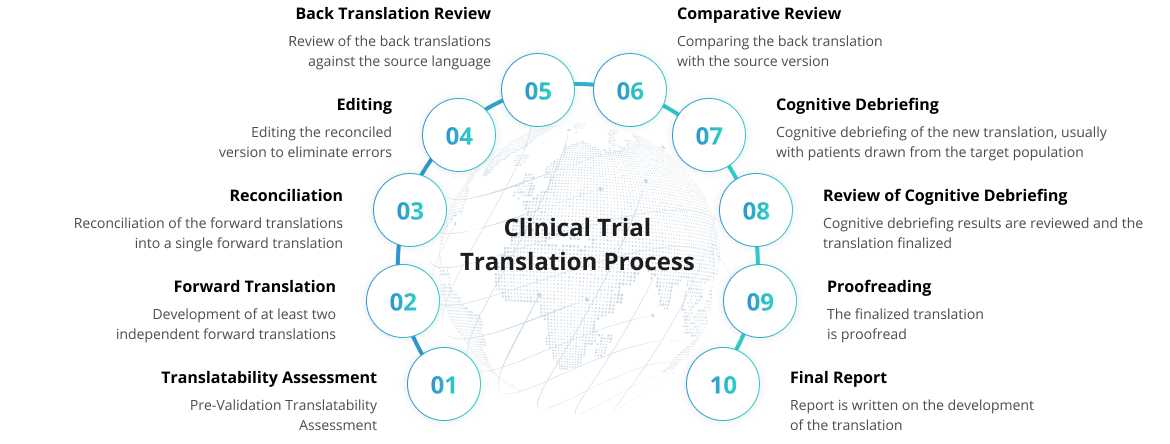
Quality Assurance for Higher Accuracy
We've set up industry standard processes to ensure accuracy
and consistency across clinical trial translations.
Enhance participant engagement with clinical trial communications, accessible in every language.
GET IN TOUCHWhat do our clients say?

Through their excellent project management system, Enago Life Sciences surpassed all my expectations and passed fairly. Given the demands of this project, it was difficult to meet my requirements but Enago Life Sciences successfully delivered my work following the timelines and high-quality standards. It is a miracle company.


Enago Life Sciences completed 400,000+ words of translation in 40 business days, all delivered on time; at the same time, the translation quality is also fully achieved to the level required by Alvogen (Lotus). We are happy and satisfied to collaborate with Enago Life Sciences. Thank you Enago Life Sciences team.


Enago Life Sciences’ translation and localization services have been highly convenient for us and I am grateful to the entire team for being so generous and sincere towards our requirements. At this point, we can almost blindly trust them with everything. They barely needed our supervision and the result delivered was exactly what we were looking for. Honestly, what else could we ask for! Thank you, Enago Life Sciences. You’re simply the best!


Working with Enago Life Sciences was the best decision, as we did not have to worry about any issue. We want to thank team Enago Life Sciences for giving us their continuous support. They went out of their way to ensure that we are satisfied with the project which impressed us as well as members at the Fukushima Medical University. Thank you all once again for doing a fantastic job on this challenging project and handling it with the same passion as we felt when compiling the book.


I have been using Enago Life Sciences services regularly for some time now and their quality of work is really good. The style and accuracy with which they translated my various documents was exactly what I expected it to be. I am really impressed with their team who worked closely with me to translate our vision into reality.

Thought Leadership
Our Memberships