Peer review plays a pivotal role in the publication of all research, assisting in maintaining its quality, ethics, and integrity. For clinical research though, peer review can begin early – during the clinical trial protocols development stage itself. This article delves into the pivotal role of peer review in shaping these protocols and how researchers can utilize it to improve study outcomes. We’ll explore how peer review is incorporated into protocol development, its significance, as well as the key stakeholders during a review. Additionally, we’ll discuss why you should publish protocols in journals and how peer review plays a role there too.
Peer Review of Clinical Trial Protocols – Why, When and How?
Clinical research, especially in fields like pharmaceuticals, requires high precision due to the stringent regulatory requirements. Research protocols are the very foundation of clinical trials, necessitating strong adherence to all ethical mandates. Protocol development is a meticulous process. It involves defining study objectives, designing methods, outlining ethical considerations, and planning statistical analyses. As high-quality protocol can ensure you have planned for all the necessary data collection, peer review fits in well during this stage.1 It can often become a critical checkpoint, usually after the initial draft of the protocol is prepared but before the study starts.
Peer review assures research integrity at the protocol development stage. This ensures your protocols are developed with minimal errors, and biases, follow all requisite ethics, and are designed to yield reliable results.2 Imagine a scenario where a research team is developing a protocol for a vaccine trial. Peer reviewers might catch issues with the participant inclusion criteria, ensuring the trial enrolls a diverse and representative group, which is critical for vaccine efficacy.
Peer review also strengthens patient safety during the trials; a well-structured protocol safeguards participants and maintains data integrity. This integrity is also crucial for regulatory approvals during the introduction of new therapies to the market.
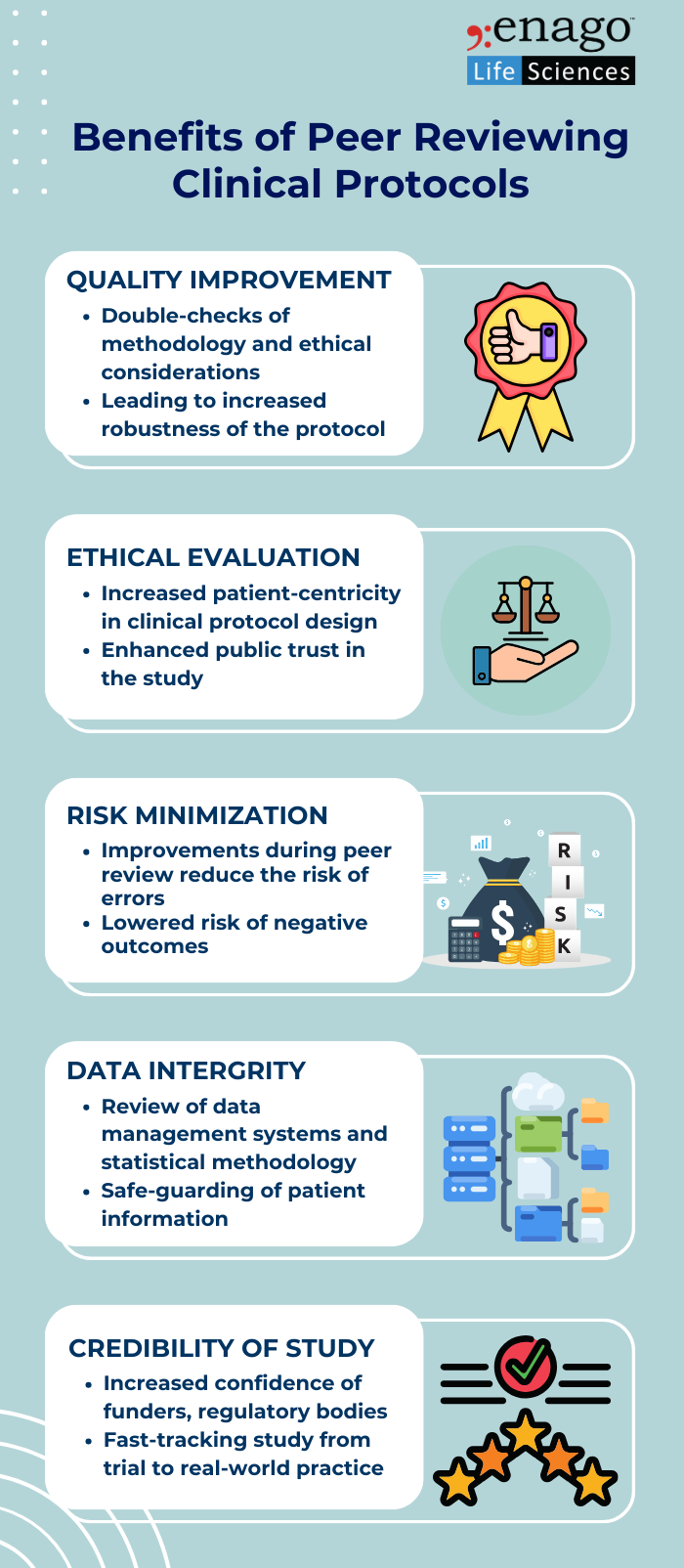
Key Stakeholders in the Peer Review Process
Peer review of clinical research protocols involves a collaborative effort among various stakeholders, each bringing their expertise to ensure the quality, ethics, and scientific rigor of the protocols.3
1. Principal Investigators
Lead researchers responsible for the protocol’s design and execution play a crucial role in the peer review process. They submit the protocol for evaluation and address the reviewers’ comments and suggestions. Their input is essential in identifying the study rationale, revisions, and improvement throughout the approval process and ensuring the protocol aligns with the study’s objectives.
2. Peer Reviewers
Peer reviewers are typically experts in the same field as the protocol under review. They provide objective and constructive feedback to assess the protocol’s scientific soundness, ethical considerations, and overall quality. These reviewers primarily ensure that the protocol is methodologically robust, ethically sound, and feasible in practice. Moreover, they may also provide insights on whether there is enough need for a proposed study and if similar studies have been conducted elsewhere.
3. Ethics Committees and Institutional Review Boards (IRBs)
These committees are responsible for evaluating the ethical aspects of the protocol, especially concerning participant safety, informed consent, and data privacy. Their input ensures that the protocol adheres to national and international ethical standards, which is vital for regulatory approvals as well.
4. Biostatisticians
This perhaps is the most common deviation from a regular article peer review performed once a study has been completed. Statisticians play a critical role in reviewing protocols, particularly in assessing the statistical methods used for data analysis. They ensure that the proposed statistical techniques are appropriate and that the sample size is sufficient to draw meaningful conclusions.
5. Funders
Many clinical studies are often funded by private investors or pharmaceutical and biotechnology companies. These companies or investors may also have their internal channels and representatives for protocol review. However, it is important to note the potential for bias in such cases.4 Due to this possible complication, ICMJE requires trial registration on an independent trial registration site, such as ClinicalTrials.gov, which performs independent review beyond funder review.5
6. Patient Advocates
In recent years, the inclusion of patient advocates in protocol peer review has gained prominence. Patient advocates provide a unique perspective by representing the interests and concerns of patients. Their involvement helps ensure that the research aligns with patient needs and values, promoting patient-centered research. They can also bring to notice any component of patient care that has been ignored or deprioritized during the protocol development, including but not limited to, support for caregivers, emotional support to patients and family, impact of patient advocacy groups.
7. Regulatory Agencies
Depending on the type of study and location, regulatory agencies such as the U.S. Food and Drug Administration (FDA) or the European Medicines Agency (EMA) may also be considered stakeholders. They review and approve clinical trial protocols involving investigational drugs or medical devices, ensuring that the research adheres to regulatory guidelines.
Aside from these, clinical data managers, project managers, safety experts, pharmacokinetics experts, formulations leads, and supply management representatives may also be involved in the peer review depending on the topic. Collaboration and communication among these stakeholders are essential for a robust and comprehensive peer review process. The collective expertise ensures that the protocol is methodologically sound, ethically rigorous, and in alignment with both scientific and regulatory standards.
The Importance of Clinical Trial Protocols Publishing in Journals
The publication of clinical research protocols in scientific journals is a crucial step that contributes to transparency, accountability, and the advancement of medical knowledge:6
1. Accountability, Transparency, and Collaboration
Protocols published in reputable journals are most often reviewed by journal editors. If the protocol has not been reviewed elsewhere, it may be subjected to regular peer review as well. This process evaluates the protocol’s scientific and ethical aspects, providing assurance that it meets the journal’s standards. This transparency promotes trust within the scientific community and among the public. It may also avoid replication of studies or even nurture a collaboration in case other researchers may be concurrently planning a similar study.
2. Nurturing Future Research
Published protocols can serve as valuable resources for other researchers in the same field. They offer guidance on study design, data collection, and analysis, helping future studies build on prior research and avoid repeating mistakes. Often, the publication may also include a detailed literature review, study rationale, or elaborate descriptions of the protocol components that are not typically readily available from the clinical trial registration.7
3. Credibility and Funding
Published protocols can enhance a research team’s credibility, making it easier to secure funding and collaboration opportunities. Funding agencies and potential collaborators can review the protocol’s scientific soundness and ethical rigor, increasing confidence in the study.
4. Impact on Clinical Practice
Protocols published in journals provide a bridge between research and clinical practice. They help clinicians stay informed about upcoming studies, allowing them to prepare for potential changes in medical practice as a result of the research. Moreover, journal publications can provide information about ongoing trials for possible treatment options or ongoing patient recruitment to practitioners.
The publication of clinical research protocols in scientific journals not only ensures transparency and accountability but also serves as a valuable resource for the scientific community. It plays a pivotal role in advancing medical knowledge and fostering ethical and rigorous research practices. As we see an enhanced acceleration of technology integration in clinical trial protocol development and management, peer review of such protocols gains center stage. Let’s explore why…
Emerging Need for Increased Peer Review of Protocols
The utilization of artificial intelligence (AI) in protocol design and development has the potential to revolutionize the field of clinical research.8,9 With cost-saving and shortened timelines at stake, the adoption of AI in the relatively safe process of protocol design is highly likely. This, in turn, indicates an even more critical need for protocol review and oversight. Reviewers must actively scrutinize AI-generated protocols due to the complex and often non-transparent decision-making algorithms. Additionally, oversight regarding ethical considerations, patient welfare and safety, regulatory compliance, and bias mitigation is also important.
These challenges underscore the importance of robust and specialized peer review processes to ensure that AI-powered protocols meet the highest standards of scientific integrity, ethics, and regulatory compliance. As AI continues to evolve and play an increasingly central role in clinical research, the need for expert and thorough protocol review becomes more critical than ever.
Author:

Dr. Gayatri Phadke
Managing Editor, Enago Academy
Connect with Gayatri on LinkedIn
References
1. Clinical Trials Toolkit | National Institute for Health and Care Research. https://www.nihr.ac.uk/. https://www.ct-toolkit.ac.uk/routemap/peer-review/ (accessed 2023-11-01).
2. Kelly, Jacalyn, Tara Sadeghieh, and Khosrow Adeli. 2014. “Peer review in scientific publications: Benefits, critiques, & a survival guide.” EJIFCC 25(3): 227.
3. Patil, Kishor P., Chandra Kumar, and Siu-Long Yao. 2023. “Peer review of a clinical trial protocol: Practical tips for regulatory medical writers, clinicians, and clinical scientists.” Medical Writing 32 (1): 20-27. https://doi.org/10.56012/fbfu9448
4. Smith, Matthew E., and Martin T.P.C. 2011. “Publishing trial protocols.” Clinical Otolaryngology 36 (5): 521–522. https://doi.org/10.1111/j.1749-4486.2011.02386.x
5. Ohtake, Patricia J., and John D. Childs. 2014. “Why Publish Study Protocols?” Physical Therapy 94 (9): 1208–1209. https://doi.org/10.2522/ptj.2014.94.9.1208
6. The Global Health Network and European & Developing Countries Clinical Trials Partnership (EDCTP) Editorial Team. 2020. “Protocol Development Toolkit”. https://doi.org/10.48060/tghn.8
7. Skogvoll, Eirik and Jo Kramer-Johansen. 2013. “Publication of clinical trial protocols – what can we learn?” Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine 21: 12. https://doi.org/10.1186/1757-7241-21-12
8. Licholai, G. 2023. “AI in Clinical Research: Now and Beyond | Forbes.” www.forbes.com. September 18, 2023. https://www.forbes.com/sites/greglicholai/2023/09/18/ai-in-clinical-research-now-and-beyond/?sh=645ae5b03c85.
9. Harrer, Stefan, Pratik Shah, Bhavna Antony, and Jianying Hu. 2019. “Artificial Intelligence for Clinical Trial Design.” Trends in Pharmacological Sciences 40 (8): 577-591. https://doi.org/10.1016/j.tips.2019.05.005


Perfect work you have done, this site is really cool with fantastic info