(Disclosure: The above featured image is generated using Notebook LM for illustrative purpose.)
Whether it’s a new cancer therapy or an innovative vaccine, the announcement of a successful clinical trial and regulatory approval feels like the final victory. It’s natural to assume that once a health technology is declared safe and effective, it will become available to every patient who needs it.
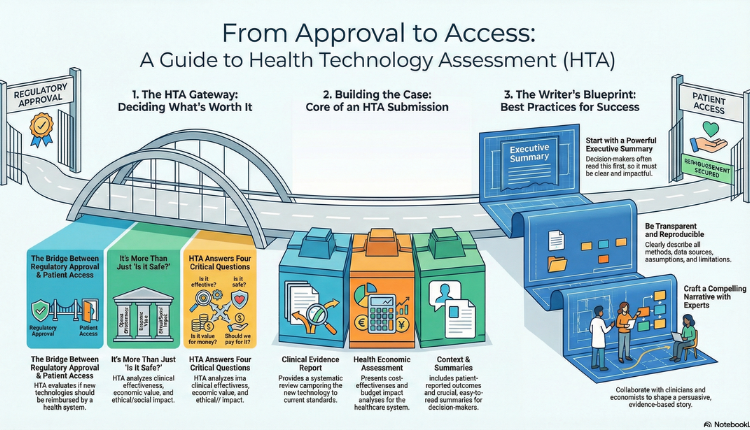
But this is where a common paradox emerges. A patient might read about a newly approved treatment, only to find out they can’t access it through their national health service. This raises a crucial question: if a new technology is approved, why can’t people get it right away? The answer lies in a vital, often invisible process that bridges scientific research and healthcare policy: Health Technology Assessment, or HTA. A “health technology” is a broad term for any intervention used to improve health, which, as defined by international experts, can be a “test, device, medicine, vaccine, procedure, program, or system.”
What is Health Technology Assessment (HTA)?
HTA is a multidisciplinary evaluation process that assesses a new technology’s true value within a specific healthcare system. It examines the clinical effectiveness, safety, economic value, ethical and social impact of a health technology. It is a cornerstone of evidence-based decision-making in healthcare systems worldwide. Technologies may include pharmaceuticals and biologics, medical devices, diagnostic tests, surgical procedures, and digital health interventions.
HTA answers critical questions such as:
- Is the technology effective compared with current standards?
- Is it safe for patients?
- Does it provide value for money?
- Should it be reimbursed by public or private payers?
HTA reports inform reimbursement decisions, pricing negotiations, clinical guideline development, and policy formulation. Evidence shows that HTA-informed reimbursement decisions help optimize population health outcomes while containing costs, particularly in publicly funded health systems.
Core Components of HTA Submissions
HTA dossiers differ by country and HTA agency (e.g., NICE (National Institute for Health and Care Excellence) in the UK, ICER in the US (Institute for Clinical and Economic Review), PBAC (Pharmaceutical Benefits Advisory Committee) in Australia, HAS (Haute Autorité de santé) in France, and local bodies in India). However, most submissions include these core elements:
A. Clinical Evidence Report
This section provides a thorough evaluation of:
- Literature review and systematic evidence synthesis
- Study selection criteria
- Clinical outcomes (efficacy, safety)
- Comparative effectiveness with current standards of care
Writers must ensure transparent methodology, reproducibility of results, and a balanced discussion of limitations.
B. Health Economic Assessment
HTA agencies often require economic evaluations such as Cost-Effectiveness Analysis and Cost-Utility Analysis. This involves:
- Model structure description
- Inputs and assumptions
- Sensitivity and scenario analyses
- Presentation of incremental cost-effectiveness ratios (ICERs)
Medical writers present economic results in tables, graphs, and narrative form while ensuring clarity for both technical and non-technical audiences.
C. Contextual and Ethical Considerations
Many HTA bodies require:
- Patient-reported outcomes
- Quality of life data
- Equity or ethical evaluations
- Real-world evidence
D. Executive Summaries and Lay Summaries
Concise summaries are crucial for broad accessibility. These must be scientifically accurate, easy to read, and tailored to stakeholders such as clinicians, payers, and patient groups.
Challenges in HTA Medical Writing
HTA writing is complex and high-stakes. Common challenges include:

Best Practices for HTA Medical Writing
To produce effective HTA documentation, follow these best practices:
1. Start With the End in Mind
Understand the HTA body’s expectations early. Use checklists and templates specific to the agency.
2. Be Transparent and Reproducible
Clearly describe methods, assumptions, data sources, and limitations.
3. Lead with Clear Executive Summaries
Decision makers often read summaries first. Ensure these are stand-alone, accurate, and impactful.
4. Use Structured Frameworks
Consistent headings, standardized tables, and well-labeled figures improve readability and review.
5. Collaborate Closely with Subject Matter Experts
Engage clinicians, statisticians, and HEOR experts throughout the writing process.
6. Anticipate and Address Potential Criticisms
Pre-emptively clarify uncertainties and provide sensitivity analyses where relevant.
7. Stay Updated
HTA methodologies evolve. Keep abreast of updates from major agencies and industry forums.
The Future of HTA and Medical Writing
Healthcare systems are shifting toward:
- Greater emphasis on real-world evidence
- Integration of patient-centric outcomes
- Use of digital health data and AI-driven analytics
- International collaboration and harmonization of HTA standards
Medical writers will play an increasingly strategic role, bridging scientific evidence with payer expectations and patient priorities.
Medical writing for HTA is not just about summarizing data. It is about crafting a compelling, evidence-based narrative that supports effective healthcare decisions. Success requires analytical depth, strategic thinking, rigorous documentation, and clear communication.
For life sciences organizations navigating the complex world of HTA, high-quality medical writing can be a decisive factor in access outcomes.
Author:

Anagha Nair
Editorial Assistant, Enago Academy
Medical Writer, Enago Life Sciences
Connect with Anagha on LinkedIn