(Disclosure: The above featured image is generated using Notebook LM for illustrative purpose.)
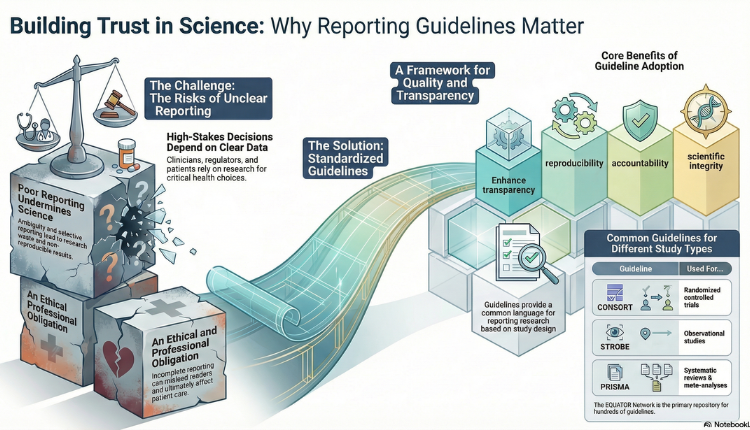
Medical communications sit at the intersection of science, healthcare, and society. They translate complex research data into information that clinicians, regulators, policy makers, and patients rely on to make critical decisions. In this high-stakes environment, how research is reported is just as important as what is reported. This is where reporting guidelines play a central role.
Reporting guidelines are structured tools which are often written in the form of checklists or flow diagrams. These guidelines help ensure medical research is communicated clearly, completely, and transparently. Their importance has grown alongside increasing concerns about reproducibility, research waste, and ethical accountability in biomedical science.
What Are Reporting Guidelines?
Reporting guidelines provide standardized frameworks for presenting research findings based on study design. The EQUATOR (Enhancing the QUAlity and Transparency Of health Research) Network serves as the primary global repository for these guidelines, covering a wide range of study types [1].
Some of the most widely used include:
| Guidelines | Study Types/Uses |
| CONSORT | Randomized controlled trials |
| STROBE | Observational studies |
| PRISMA | Systematic reviews and meta-analyses |
| SPIRIT | Study protocols |
| PRISMA-P | Study protocols |
| STARD | Diagnostic and prognostic studies |
| TRIPOD | Diagnostic and prognostic studies |
| CARE | Case reports |
| ARRIVE | Animal studies |
| SQUIRE | Quality improvement research |
| CHEERS | Economic evaluations |
| SRQR | Qualitative research |
| COREQ | Qualitative research |
| ACCORD | All consensus-based studies |
| GAMER | Transparency in studies involving Artificial Intelligence/Machine Learning |
Together, these guidelines provide a common language for medical communication across disciplines, journals, and regions.
Enhancing Transparency and Reproducibility![Enchancing Transparency & Reproducibility]()
One of the most important contributions of reporting guidelines is improved transparency. By specifying the minimum essential information that must be reported, guidelines reduce ambiguity, selective outcome reporting, and “spin” in research publications [2,3].
Transparent reporting directly supports reproducibility, a cornerstone of credible medical science. When methods, analyses, and results are described consistently and in sufficient detail, other researchers can replicate or build upon the work with confidence [2,4]. Without this clarity, even well-designed studies may lose scientific and societal value.
Strengthening Medical Communications Across Formats
 Medical communications extend far beyond journal articles. They include conference abstracts, posters, clinical trial registries, plain-language summaries, educational materials, and digital content. Reporting guidelines help ensure consistency and accuracy across all these formats [5,6].
Medical communications extend far beyond journal articles. They include conference abstracts, posters, clinical trial registries, plain-language summaries, educational materials, and digital content. Reporting guidelines help ensure consistency and accuracy across all these formats [5,6].
For journals and conferences, standardized reporting improves comparability across publications and streamlines editorial and peer-review processes [2,3,7]. Reviewers can focus on scientific merit rather than missing information, leading to more efficient and meaningful evaluations.
For clinicians and policymakers, guideline-driven reporting ensures that evidence is reliable and applicable to real-world decision-making [5,8].
Ethical and Professional Responsibility
 Poor reporting is not just a technical issue but also an ethical one. Incomplete or unclear reporting can mislead readers, distort evidence synthesis, and ultimately affect patient care [4]. From this perspective, transparent reporting is a professional obligation rather than an optional best practice.
Poor reporting is not just a technical issue but also an ethical one. Incomplete or unclear reporting can mislead readers, distort evidence synthesis, and ultimately affect patient care [4]. From this perspective, transparent reporting is a professional obligation rather than an optional best practice.
Reporting guidelines promote:
- Accountability, by holding researchers, medical writers, and journals to defined standards [2,4]
- Bias reduction, by discouraging selective reporting and exaggerated claims [3,8]
- Integrity, by ensuring that research funded and conducted for societal benefit is communicated responsibly [4]
They also protect institutions and sponsors from legal and reputational risks by aligning communications with regulatory and ethical expectations [5,7,8].
Educational Value and Career Development
 Beyond their regulatory and ethical roles, reporting guidelines are powerful educational tools. They help early-career researchers and medical communicators learn how to structure manuscripts, interpret results responsibly, and communicate science effectively [2,3,7].
Beyond their regulatory and ethical roles, reporting guidelines are powerful educational tools. They help early-career researchers and medical communicators learn how to structure manuscripts, interpret results responsibly, and communicate science effectively [2,3,7].
In medical communication agencies and publishing teams, guidelines provide a shared framework that supports collaboration among writers, statisticians, clinicians, and designers [6]. For professionals entering the MedComms field, familiarity with guidelines is often a foundational skill for career success.
Global Standards and Future Directions

Reporting guidelines are now recognized and adopted internationally, creating global consistency in how medical research is communicated [2,3]. Many are open access, improving equity for researchers and communicators in resource-limited settings [4].
Looking ahead, several trends are shaping the future role of reporting guidelines:
- Digital and AI integration, embedding guidelines into submission systems and content-development tools [2,4,8]
- Expansion to new formats, including patient-reported outcomes, qualitative research, and digital media [2,6]
- Continuous updates, ensuring relevance as methodologies, regulations, and communication platforms evolve [3,7]
Despite their widespread availability, challenges remain in implementation, dissemination, and consistent uptake. This highlights the need for ongoing education and mechanisms of audit and feedback [3,4].
Conclusion
Reporting guidelines matter in medical communications because they safeguard clarity, credibility, and ethical integrity. They ensure that research findings are not only published, but also understood, trusted, and applied in ways that improve healthcare outcomes.
In a field where communication can influence clinical practice, regulatory decisions, and patient lives, reporting guidelines are not only checklists but an essential tool for responsible, high-quality medical communication.
References
- EQUATOR Network. Enhancing the QUAlity and Transparency Of Health Research. EQUATOR Network. Accessed January 16, 2026. https://www.equator-network.org
- Katyayan M. Reporting guidelines: Elevating standards in scientific communication. J Indian Prosthodont Soc. 2025 Jan 1;25(1):1-2.3. Enago Academy. The Top Six Guidelines for Reporting Medical Research.
- Schlussel MM, Sharp MK, de Beyer JA, Kirtley S, Logullo P, Dhiman P, et al. Reporting guidelines used varying methodology to develop recommendations. J Clin Epidemiol. 2023; 159:246-256.
- Moher D. Reporting guidelines: doing better for readers. BMC Med. 2018; 16:233.
- St Giles Medical. What Is Medical Communications – And Why Does It Matter? St Giles Medical. Published May 1, 2025. Accessed January 6, 2026. https://www.stgmed.com/post/what-is-medical-communications-and-why-does-it-matter
- Whitaker MW. From SciComms to MedComms: An Intro to Medical Communications. PLOS ECR Community. Published April 8, 2025. Accessed January 6, 2026. https://ecrcommunity.plos.org/2025/04/08/from-scicomms-to-medcomms-an-intro-to-medical-communications
- Enago Academy. Published 2023. Accessed January 16, 2026. https://www.enago.com/academy/top-six-guidelines-for-reporting-research
- Able AG. Navigating Regulatory Compliance in Medical Communications. Able AG. Published January 3, 2026. Accessed January 6, 2026. https://www.ableag.org/navigating-regulatory-compliance-in-medical-communications
Author:

Sweaksha Langoo (MSc. Molecular Biology and Biochemistry)
Scientific Writer – Enago Life Sciences
Connect with Sweaksha on LinkedIn