(Disclosure: The above featured image is generated using Notebook LM for illustrative purpose.)
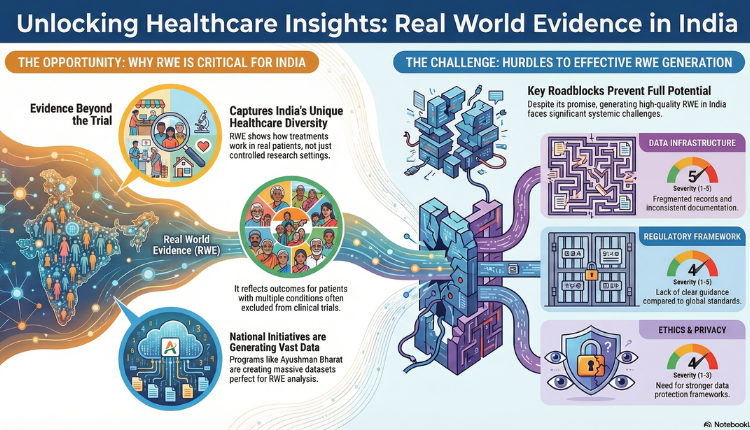
Today, medical decision-making demands evidence that portrays real-life events outside controlled research settings. Though randomized controlled trials are the cornerstone for evaluating the efficacy and safety of any treatment, they may not necessarily represent reality as it exists in everyday clinical practice. This realization has driven increasing attention to Real World Evidence, especially for countries with a diverse healthcare delivery setup, such as India.
RWE provides insights into how treatments work in real world environment, among broader group of patients, and over longer time periods than those in idealized trial environment. When positioned properly, RWE can fill an important gap in evidence that exists in relation to clinical trials in India.
Understanding Real-World Evidence?
RWE is clinical evidence about a medical product’s usage and potential benefits or risks generated by analysing real-world data (RWD) [1]. The sources of RWD include electronic health records, patient medical charts, disease registries, insurance claims, pharmacy records, and patient-reported outcomes [2].
Unlike clinical trials, which operate under predefined protocols and strict eligibility criteria, RWE reflects how treatments are used in practice. It helps answer practical questions such as how well a therapy works in real patients, how long patients remain on treatment, and what outcomes are observed over time.

Why is RWE Important in India
- India’s healthcare landscape is highly diverse — spanning geography, access, socio-economic status, and disease burden
- Patients often present with multiple co-morbidities and varied disease severity, which are under-represented in clinical trials
- Treatment adherence patterns differ widely across regions and populations [3]
- RWE reflects outcomes in routine clinical settings, capturing this complexity more accurately
- It generates more representative evidence for Indian patients, enhancing relevance for everyday clinical decisions
- RWE captures diversity better as it reflects outcomes in routine clinical settings. By providing more representative evidence for the Indian population, it also makes the evidence more applicable to a day-to-day clinical decision.
Current Landscape of RWE in India
- India faces a dual disease burden: communicable diseases and rising non-communicable conditions like diabetes, cardiovascular disease, cancer, and chronic respiratory disorders [3]
- Ayushman Bharat–Pradhan Mantri Jan Arogya Yojana (AB-PMJAY) — one of the world’s largest government-funded health insurance schemes, generating vast real-world data
- Data sources include insurance claims, hospitalization records, treatment pathways, and healthcare utilization across public and empaneled private hospitals
- The datasets reflect routine clinical practice across diverse geographies and populations, offering insights into treatment effectiveness, safety, access, and cost
- Integration with Ayushman Bharat Digital Mission (ABDM) enables longitudinal data capture via unique health IDs and electronic health records. This digital infrastructure helps overcome challenges of fragmented and non-standardized healthcare data in India.
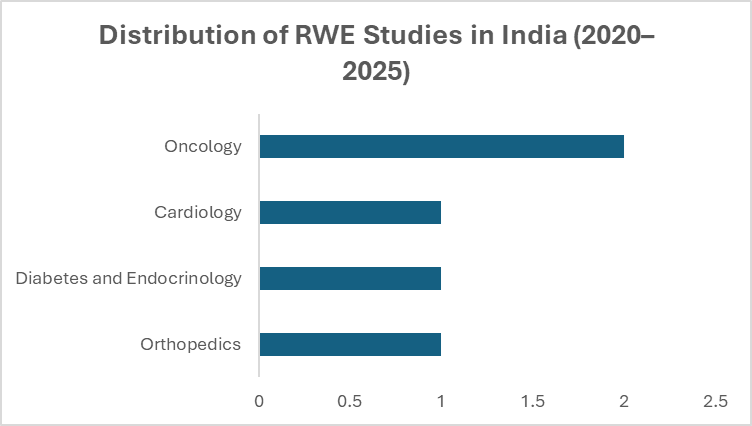
Published literature reports very small number of RWE studies conducted across India between 2020–2025 [4-8].
Challenges to Effective RWE Generation
| Category | Key Issues | Severity (1–5) |
| Data Infrastructure | Not centralized, fragmented records, inconsistent follow-up, insufficient documentation [9] | 5 |
| Regulatory Framework | Limited clarity, lack of guidance compared to global frameworks | 4 |
| Ethics & Privacy | Partial safeguards under IT Act (2000/2008) & Personal Data Protection Bill (2019), need stronger frameworks [11] | 4 |
| Methodology | Lack of clarity on acceptable RWE methods, need hybrid designs & robust planning [2] | 4 |
| Stakeholder Awareness | Variable awareness and acceptance among stakeholders | 3 |
| Bias & Transparency | Concerns about bias, ethics, and transparency | 4 |
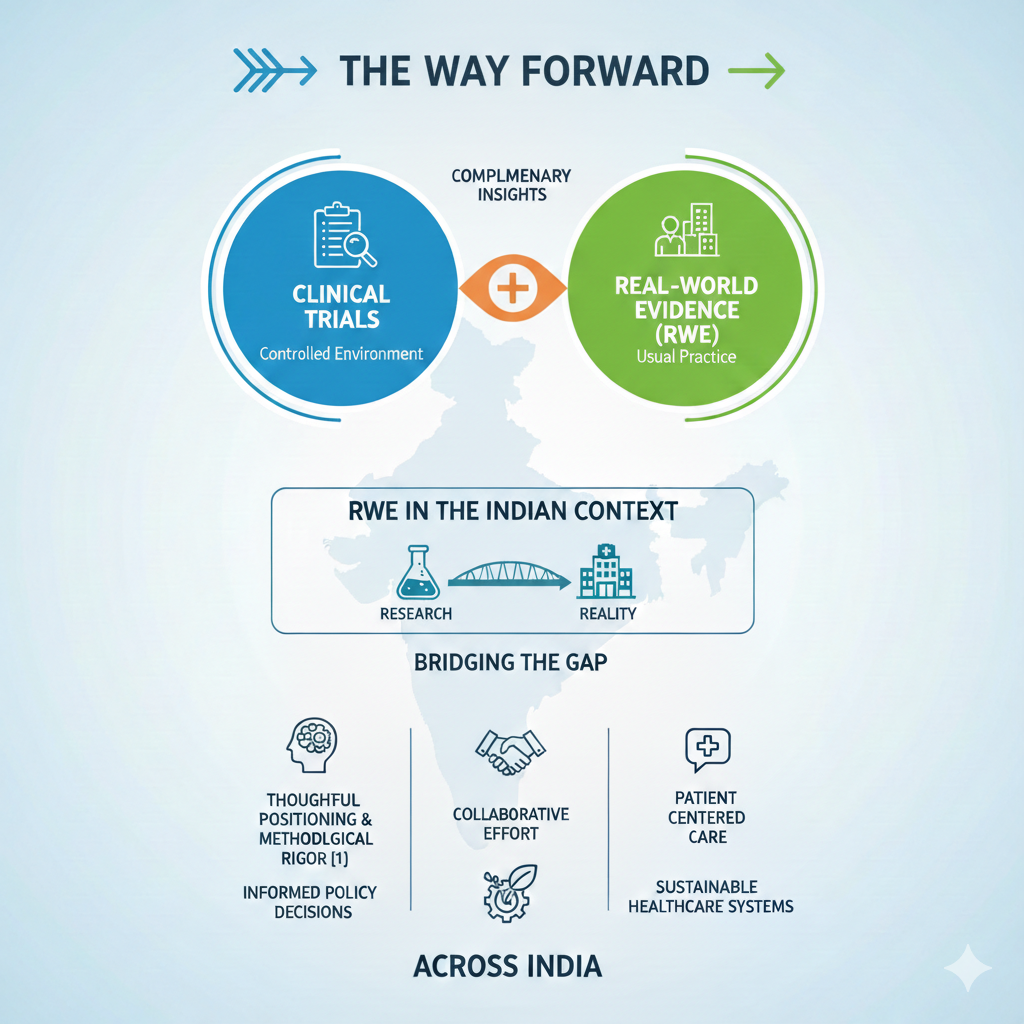
Strengthening the Role of RWE
The strength of RWE also depends on the quality of data it holds. Good studies that have defined research questions, relevant comparators, and valid analytic approaches can deliver valuable and trusted insights [10]. Enhancing the digital infrastructure, facilitating collaboration among stakeholders such as the health care delivery sector, academia, industry, and the government could lead to better quality studies using real-world evidence.
References
- US Food & Drug Administration (FDA) Real-world evidence https://www.fda.gov/science-research/science-and-research-special-topics/real-world-evidence(2023), Accessed 25th Sep 2023.
- Bhatt A. Conducting real-world evidence studies in India. Perspect Clin Res. 2019 Apr–Jun;10(2):51–56. doi:10.4103/picr.PICR_8_19.
- Elsaka O. Real-world evidence vs. clinical trials: outcome disparities in valve interventions. MGM J Med Sci. 2025 Apr–Jun;12(2):352–362. doi:10.4103/mgmj.mgmj_70_25.
- Eashwar P, Yadlapalli DC, Gullipalli M. Experience with palbociclib in metastatic breast cancer patients managed under a government health scheme at a cancer care center in Southern India. Cureus. 2024 Sep 28;16(9):e70394. doi:10.7759/cureus.70394.
- Noronha V, Abraham G, Patil V, Joshi A, Menon N, Mahajan A, et al. A real-world data of immune checkpoint inhibitors in solid tumors from India. Cancer Med. 2021 Mar;10(5):1525–1534. doi:10.1002/cam4.3617
- Le Ruz R, Leroux L, Lhermusier T, Cuisset T, Van Belle E, Dibie A, et al. Outcomes of transcatheter aortic valve implantation for native aortic valve regurgitation. EuroIntervention. 2024 Sep 2;20(17):e1076–e1085. doi:10.4244/EIJ-D-24-00339
- Panikar V, Joshi SR, Deogaonkar N, Vadgama J, Nasikkar N, Kamat T, et al. Efficacy of SGLT2 inhibitors as the fifth drug in the management of type 2 diabetes mellitus in Asian Indians not controlled with at least 4 oral antidiabetic drugs. J Assoc Physicians India. 2018 Dec;66(12):46–49
- Chaudhari Y, Thombare K. Perioperative outcomes of simultaneous bilateral total knee arthroplasty in elderly Indian patients: a retrospective study. J Orthop Case Rep. 2025 Jul;15(7):288–294. doi:10.13107/jocr.2025.v15.i07.5852.
- Goyal RK, et al. Real-World Evidence in India: Opportunities and Challenges. Perspectives in Clinical Research. 2019.
- Kale S, Vatkar A, Gehilot O, Shyam A. Real-world evidence: Methodologies for integrating real-world data into clinical research frameworks. J Orthop Case Rep. 2025;15(10):6–9. doi:10.13107/jocr.2025.v15.i10.6138.
- Real World Evidence Council Working Group, Indian Society for Clinical Research. Real world evidence (RWE): an Indian perspective. Mumbai: Indian Society for Clinical Research; 2022. //www.iscr.org/files/Article/1156679472_Position-paper_RWE_10-May-202.pdf
Author:

Manasvi Joshi, Msc in Biotechnology
Senior Scientific Writer 1, Enago Life Sciences
Connect with Manasvi on Linkedin
Reviewer:

Dhanya Mukundan, MDS (Oral Medicine and Radiology)
Expert Scientific Writer, Enago Life Sciences
Connect with Dhanya on LinkedIn