“If anyone wishes to write a clear style, let them be clear in their thoughts” Johann Wolfgang van Goethe Manuscript writing is the art of presenting complex and huge scientific data in a simple, concise, and presentable story format. Development of a scientific manuscript requires profound knowledge across therapy areas and expertise in presenting complex data as a short, simple, and succinct story. It requires an understanding of the target journals and publication guidelines.
Scientific publications aid in disseminating clinical data to the scientific community and maintain transparency of the research outcome. Publication of manuscripts in scientific journals generates peer-reviewed, citable references. Whereas, presentation of abstracts, posters, oral presentations at scientific congresses ensures availability of data in the public domain much ahead of the peer-reviewed publication. Amongst scientific publications, the manuscript is a more descriptive way of presenting research to the external world. The key objective of communication through a manuscript is to present facts based on scientific data and research, by following the Good Publication Practice (GPP3) guidelines that ensures data integrity and publication ethics.
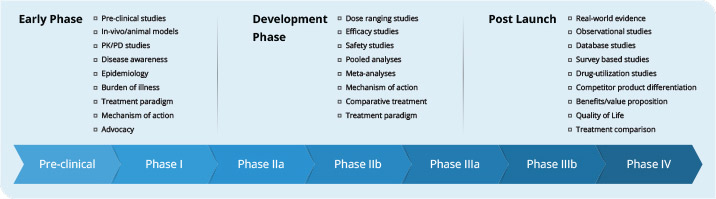
The publication plan for a drug is developed based on the development stage of the molecule, gaps identified in the literature, brand strategic imperatives and messaging the target audience. There are different types of scientific manuscripts developed to communicate data during the drug development phases as well as later in the life-cycle management (Figure 1). Manuscripts developed at the early phase of drug development covers preclinical, PK-PD studies, and topics related to the mechanism of action, disease awareness, advocacy etc. Through Phase I–III clinical trials, primary or secondary manuscripts focusing on dose regimen/efficacy/ safety objectives of a clinical study, pooled analysis, treatment comparisons etc. are developed. In the late phase IV and product life-cycle management; manuscripts on real-world evidence, surveys, observational studies, health economics, competitor product differentiation, treatment paradigm, etc., are developed.
Figure 1. Manuscript planning through drug life-cycle
Manuscript writing is the art to present complex scientific data in a story format. To develop a good manuscript, the medical writer should have a flair for writing, therapeutic area expertise, understanding of clinical trials and drug development process, and project management skills. Here, we discuss the tips to develop a manuscript focusing on clinical research.
Tips to develop a manuscript:
- As you get started with manuscript writing, first things first get familiarized with the topic, read, read and read…
- Familiarize yourself with the data and identify gaps, if any. Perform extensive literature search and extract relevant information to supplement the data.
- As you assimilate information to develop a story outline/skeleton around objectives and key messages, follow a logical structure.
- Full manuscript should have a story with a clear message based on a logical sequence of thoughts supported by text, tables, figures, and references.
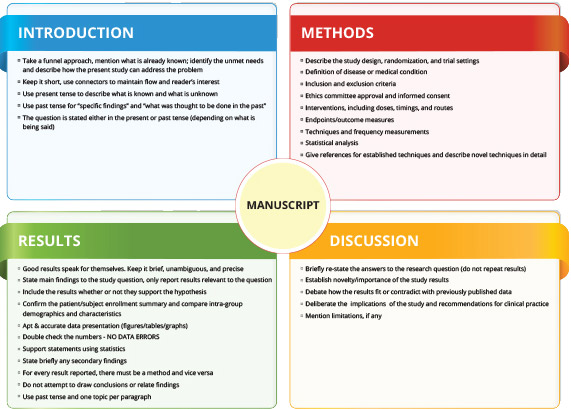
- Manuscript writing broadly follows the IMRAD (Introduction, Methods, Results, And Discussion) structure (Figure 2). The story must be woven in the context of established literature (Introduction), unmet needs and the research question (Objectives), procedures performed (Methods), the research outcome, (Results) and the research outcome implications (Discussion). Ensure uniform flow of objectives and associated parameters throughout different sections methods, results, and discussion. A small paragraph concluding the research outcome can also be added towards the end.
pertaining to the manuscript development. Gather all the other information related to the source material, target audience, probable key messages, journals, timelines, authorship, affiliations, disclosures, conflict of interest, acknowledgments, review, and signoff process etc.
Figure 2: Tips to develop IMRAD structure
Follow the 3 C’s of writing

- Figures and Tables (max 6–8) should be self-explanatory as a standalone image/table and follow the same sequence as mentioned in the text. Use brief, informative legends, titles, and footnotes. Ensure consistent abbreviations, variables, units, symbols, and colors as per journal specifications.
- References should be styled consistently as per journal guidelines, use latest references wherever possible.
- Perform plagiarism and copyright check.
- Follow journal guidelines – instructions to the authors, and format the manuscript as per journal specifications.
- Follow standard reporting guidelines for the study type e.g., CONSORT checklist for randomized trials. Refer the EQUATOR network to access the relevant
checklists. . - Review your work thoroughly, once done revisit it with a fresh mind the next day, it will definitely help you improve your manuscript. Get it reviewed by a peer or
a friend, who can spot gaps and make it better - Perform a mock journal submission and prepare your checklist for the journal submission package.
Enago Life Sciences is a medical writing company with specialization in medical communications services. We provide variety of publication services including manuscript writing across all phases of the drug development, preclinical to post-launch, and product life-cycle management. We work closely with the authors/clients to develop the manuscript from initiation up to final approval. A manuscript is developed within a typical turnaround time of 12 weeks (kickoff – submission package). Our highly qualified, experienced team, with an excellent knowledge across therapy and industry standards, develops the manuscript as per the key messages, journal guidelines, and Good Publication Practice.


I have read so many articles or reviews on the topic of the blogger lovers except this piece of writing is in fact a
pleasant post, keep it up.
I really loved this particular post. Really thorough and
easy to understand.
I check out your blog fairly often as I really like your blogging method.
I shared this on my Facebook and my followers was fascinated with it too.
Keep up the good work!
Greate article. Keep posting such kind of info on your site.
Im really impressed by your blog.
Hello there, You’ve done a fantastic job. I’ll certainly digg it and personally suggest to my friends.
I’m sure they’ll be benefited from this website.
WOW just what I was searching for. Came here by searching for modern canvas art prints
excellent issues altogether, you simply gained a logo new reader.
What would you suggest in regards to your publish that
you just made some days ago? Any sure?
Magnificent goods from you, man. I’ve understand your stuff
previous to and you’re just extremely wonderful. I actually like what
you have acquired here, certainly like what you are stating and the way in which you say it.
You make it entertaining and you still take care of to keep it wise.
I can’t wait to read much more from you. This is
really a great web site.
Thanks for a marvelous posting! I certainly enjoyed reading
it, you are a great author.I will remember to bookmark your
blog and will eventually come back very soon. I want to
encourage you continue your great work, have a nice day!
you are actually a just right webmaster. The web site loading speed is incredible.
It seems that you are doing any unique trick. Also, The contents are masterwork.
you’ve done a wonderful activity in this matter!
It is in reality a nice and helpful piece of info. I am satisfied that you shared this helpful information with us.
Please keep us up to date like this. Thank you for
sharing.
It’s an amazing post for all the web people; they will take advantage from it I am sure.
Hi there, I check your new stuff on a regular basis.
Your writing style is awesome, keep up the good work!
Appreciate the recommendation. Will try it out.
It’s going to be ending of mine day, but before finish
I am reading this impressive article to increase my know-how.
Thankfulness to my father who told me on the topic of this blog, this webpage is really remarkable.
Thanks for sharing your thoughts. I truly appreciate
your efforts and I will be waiting for your next write
ups thanks once again.
It’s really very difficult in this full of activity life to listen news on Television, therefore I simply use world wide web for that
reason, and obtain the most up-to-date information.
This piece of writing presents clear idea designed for
the new people of blogging, that truly how to do blogging.
Your style is unique in comparison to other folks I have
read stuff from. Thanks for posting when you have the opportunity, Guess I’ll just bookmark this blog.
Quality articles or reviews is the secret to attract the viewers to pay a visit the web page,
that’s what this website is providing.
Hello Dear, are you really visiting this web site daily, if
so after that you will absolutely obtain good experience.
Thank you for sharing your info. I really appreciate your
efforts and I am waiting for your further post thanks once again.
Yeah bookmaking this wasn’t a high risk decision great post!
Beautiful and even precise in fact. I for instance the content understanding in home-page. Also the several options designed for blog and even full-width sites. Looks like this is usually a great area for young adults.