Key Takeaways
-
Orphan drugs are scientifically powerful—but financially overwhelming.
-
Access is nearly nonexistent in LMICs due to high costs and import barriers.
-
Ethical tensions include affordability, fairness, and healthcare prioritization.
-
Policy solutions: value-based pricing, public-private R&D, and income-based pricing.
-
Better data and real-world evidence are essential for fair pricing and access.
-
A global compact is needed to align innovation with health equity.
Executive Summary
Orphan drugs—treatments for rare diseases—offer life-changing breakthroughs but at extreme costs, often over $300,000 to $4 million per patient. This creates a global access crisis: while innovation surges, affordability and equity lag, especially in low- and middle-income countries where most patients are excluded. As pricing models strain healthcare systems, urgent reforms are needed to balance innovation with access. Solutions like value-based pricing, transparent R&D, and global tiered pricing offer a path forward. The WHO’s Rare Disease Framework calls for coordinated action to make rare disease care more inclusive and sustainable.
The Ethical Dilemma of Orphan Drugs
In the realm of modern medicine, few challenges are as complex—and as ethically fraught—as the treatment of rare diseases. Affecting 3.5% to 5.9% of the global population, rare diseases present a dual narrative: one of groundbreaking scientific innovation, and another of escalating economic strain. At the heart of this tension are orphan drugs—high-cost therapies designed for conditions that afflict fewer than 1 in 2,000 people.
These therapies hold remarkable promise, often delivering life-altering, and in some cases curative, outcomes for patients who previously had no treatment options. Yet with annual prices often exceeding $300,000—and in some cases, reaching as high as $4 million—these treatments expose a fundamental paradox: how can society support the cost of compassionate innovation without compromising healthcare equity?
The Economics of Rarity: A Market Built on Exceptions
Yet this growth is built on fragile economic underpinnings. The development of an orphan drug can cost up to $1.5 billion—similar to more common medications—but the return on investment must be extracted from far smaller patient populations. This dynamic has created an “inverse pricing model”—the fewer the patients, the higher the price. Gene therapies for hemophilia B and spinal muscular atrophy can exceed $3.5 million per patient, while CAR T-cell therapies frequently top $400,000 per infusion.
Companies often justify these prices by citing scientific complexity and long-term cost savings—such as reduced hospitalizations or one-time cures. However, critics argue that industry-reported R&D expenditures are often opaque and potentially inflated.
Structural Barriers in Low- and Middle-Income Countries (LMICs)
Orphan drugs are largely inaccessible in low- and middle-income countries (LMICs). Studies show they have a very limited presence in middle-income countries and are virtually absent in low-income countries, leaving most patients with rare diseases in these regions without access to life-saving treatments.
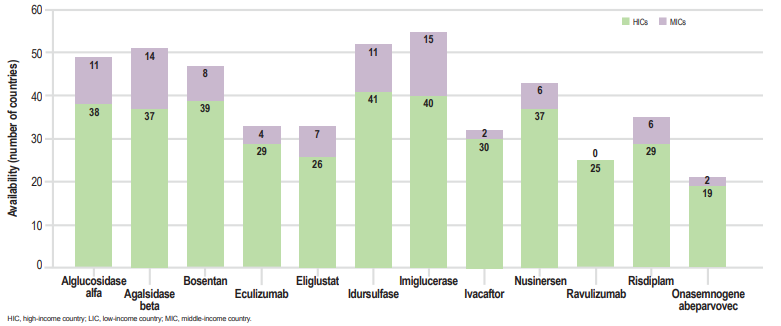
Orphan Drug Availability Across Country Income Groups. This graph highlights the limited availability and delayed access to orphan drugs in low- and middle-income countries (LMICs), underscoring urgent policy and pricing reforms. Source: Brown D, Orphan Drugs in LMICs, ISPOR Europe 2022.
The financial burden is especially crushing in LMICs. In India, for example, therapies like trientine for Wilson’s disease or eliglustat for Gaucher’s disease cost more than ₹1.6 crore (~$192,000) annually. As a result, more than 95% of patients are effectively excluded from access. Such structural inequities render even approved therapies inaccessible, reinforcing the need for global strategies that center both innovation and inclusivity.
The following table helps clarify how structural economic barriers in the orphan drug market translate directly into steep costs and limited access for patients, especially in low- and middle-income countries:
| Barrier | Economic Mechanism | Impact on Patients |
| Limited economies of scale | High fixed R&D costs spread across few users | Annual costs > $200,000 |
| Monopoly pricing | Regulatory exclusivity for orphan drugs | No competition; no generics |
| Importation markups | Lack of local production | 300–500% increase in end-user costs |
The Ethical Crossroads: Innovation vs. Access
As healthcare budgets tighten and gene therapies proliferate, decision-makers face existential questions: Should a treatment for 100 people cost 100 times more per capita than one for 10,000? What happens when cures exist, but only for those who can afford them?
The ethical dilemmas are stark:
- Financial toxicity: A recent survey found that even with insurance, about one-quarter of rare disease patients in the U.S. face annual out-of-pocket healthcare costs exceeding $3,000, with many also reporting significant financial strain, barriers to care, and poor health-related quality of life as a result.
- Geographic disparities: More than 95% of approved orphan drugs remain unavailable in LMICs.
- Intergenerational tension: High-cost pediatric therapies raise difficult questions about opportunity costs and public spending priorities.
Policy Innovations and Market-Based Solutions
Addressing the orphan drug paradox requires more than philanthropy—it demands structural reform. Promising models include:
- Value-Based and Performance-Linked Pricing
Tying payment to clinical outcomes—such as installment-based payments or pay-for-performance contracts— can ensure that pricing reflects actual clinical outcomes while distributing financial risk more equitably among payers.
- Transparent R&D Costing
Mandatory public disclosure of R&D expenditures and subsidies could reduce price inflation and enable more informed negotiations.
- Collaborative R&D and Risk Sharing
Initiatives like the NIH’s Bespoke Gene Therapy Consortium demonstrate how public-private partnerships can reduce development risk and cut prices by up to 40%.
- Global Procurement and Tiered Pricing
Multinational joint procurement agreements can pool demand and enhance bargaining power. Equity-based pricing—like the pricing of Zolgensma at $420,000 in Thailand vs. $2.1 million in the U.S.—illustrates the feasibility of adapting prices to local economic contexts.
- Clinical Trial Innovation
Innovative trial designs (e.g., basket trials) and digital recruitment platforms can streamline development and expand access while lowering costs.
Evidence Gaps and the Need for Better Data
Economic evaluations of orphan drugs remain sparse and methodologically inconsistent. A 2024 umbrella review found that most cost-effectiveness studies were of low or moderate quality. Without robust data on disease progression, indirect costs, and long-term outcomes, policymakers struggle to justify high prices or assess long-term value.
Real-world evidence (RWE) must be prioritized to fill these gaps. Public registries, standardized outcomes, and post-market surveillance are essential tools for future policy coherence.
Toward a Global Compact on Rare Disease Innovation
The World Health Organization’s 2025 Rare Disease Framework calls for coordinated international action, emphasizing ethical pricing, inclusive access, and sustainable innovation. A new paradigm—rooted in risk-sharing partnerships, transparent cost reporting, and equity-based pricing—is not just desirable but essential.
The proposed framework could incorporate
- Governments co-funding early R&D in exchange for price caps post-approval
- Public disclosure of RWE to support better reimbursement decisions
- Global differential pricing based on national income and disease burden
- Offering zero‑interest EMI plans for high‑cost drugs, allowing patients to pay small instalments monthly instead huge amounts upfront
The orphan drug economy reflects the best and worst of biomedical progress: miraculous breakthroughs shadowed by mounting inequities. As the market races in value, the stakes are clear. Left unchecked, pricing models risk exclusion—curing disease for some while leaving others behind. The path forward must blend science with conscience. Balancing the imperatives of innovation, access, and equity will require bold policy, collaborative vision, and moral clarity.
References:
- AgencyIQ. 2024. “New Regulatory Playbook from FDA-Supported Bespoke Gene Therapy Consortium Aims to Accelerate Development.” AgencyIQ Blog. https://www.agencyiq.com/blog/new-regulatory-playbook-from-fda-supported-bespoke-gene-therapy-consortium-aims-to-accelerate-development/.
- Flanigan, Kimberly M., Holly L. Peay, Samantha L. Parker, and Barbara B. Biesecker. 2023. “Understanding Patient Experiences with Rare Diseases: A Qualitative Review of the Literature.” Journal of Genetic Counseling 32 (4): 746–758. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10476732/.
- Forsythe, Laura P., Elizabeth A. DiNenno, Sarah A. Birken, and Gila Neta. 2024. “Enhancing Patient-Centered Outcomes Research through Partnership with Rare Disease Communities.” Health Affairs 43 (1): 53–62. https://pubmed.ncbi.nlm.nih.gov/39333302/.
- Office of Health Economics. 2024. “The Use of Pay-for-Performance for Drugs: Can It Improve Incentives for Innovation?” OHE. https://www.ohe.org/publications/use-pay-performance-drugs-can-it-improve-incentives-innovation/.
- Rubin, Richard. 2024. “Democratic Bill Would Require Disclosure of Pharma R&D Expenditures.” Thomson Reuters Tax & Accounting News. https://tax.thomsonreuters.com/news/democratic-bill-would-require-disclosure-of-pharma-rd-expenditures/.
- Towse, Adrian, Jorge Mestre-Ferrandiz, and Mike Drummond. 2024. “The Use of Pay-for-Performance for Drugs: Can It Improve Incentives for Innovation?” PharmacoEconomics 42: 455–467. https://link.springer.com/article/10.1007/s40273-024-01370-2.
- World Health Organization. 2025. “Seventy-Eighth World Health Assembly—Daily Update, 24 May 2025.” WHO. May 24, 2025. https://www.who.int/news/item/24-05-2025-seventy-eighth-world-health-assembly—daily-update–24-may-2025.
Author:

Rebecca D’souza, PhD.
Associate Content Expert, Enago Academy
Connect with Rebecca on LinkedIn