In the realm of medical communications, robust and accurate literature reviews are the backbone of high-quality content development. Whether it’s for medical writing, strategic communications, regulatory documents, or scientific publications, the choice of databases to conduct a comprehensive literature review is crucial. PubMed and Embase are two of the most widely used databases for medical literature searches, and while they often overlap, they serve distinct purposes and offer unique strengths. Understanding the nuances of each is key to maximizing the effectiveness of your literature review and ensuring a comprehensive analysis.
PubMed: The Gold Standard for Biomedical Research
PubMed, maintained by the National Library of Medicine (NLM), is a free resource offering access to over 37 million citations and abstracts of biomedical literature. It primarily indexes the MEDLINE database, which covers peer-reviewed journals in medicine, nursing, dentistry, healthcare systems, and more. It also includes articles from PubMed Central (PMC) and books in the NCBI Bookshelf, adding additional layers of valuable content to the search process.
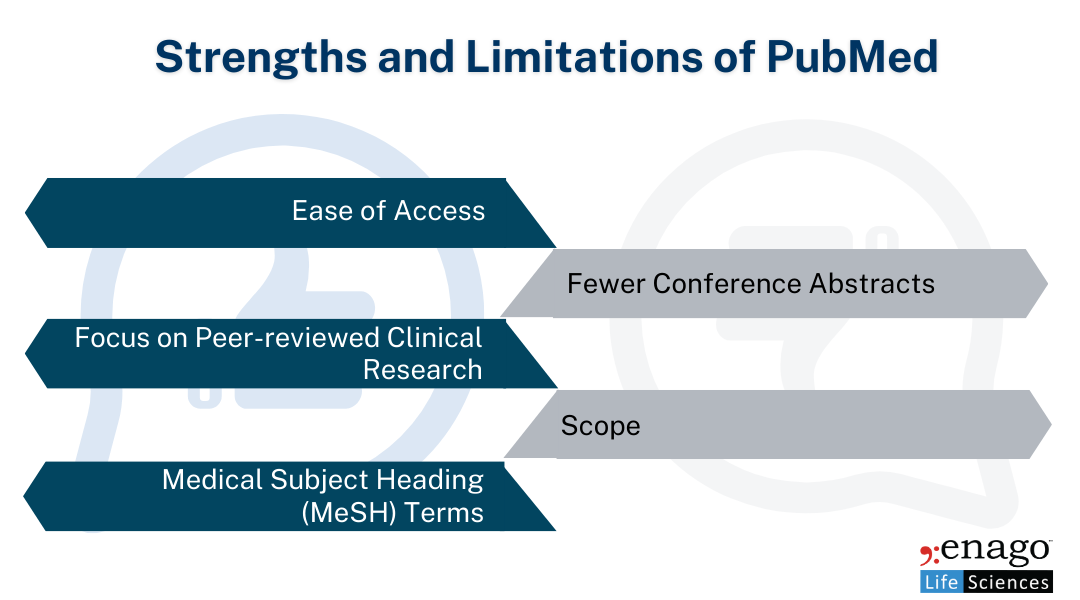
Strengths of PubMed:
1. Ease of Access
PubMed is free, easy to use, and widely recognized, making it accessible to medical writers, researchers, and healthcare professionals across the globe.
2. Focus on Peer-Reviewed, Clinical Research:
PubMed’s indexing largely focuses on high-quality, peer-reviewed clinical research. For medical communications professionals, this is crucial when writing manuscripts, regulatory documents, or creating scientific narratives that require trustworthy sources.
3. MeSH Terms (Medical Subject Headings):
PubMed’s controlled vocabulary via MeSH allows for precise searches. This feature helps writers and researchers filter studies efficiently, especially when exploring specific clinical questions or looking for highly relevant systematic reviews.
Limitations of PubMed:
1. Fewer Conference Abstracts:
PubMed does not index as many conference abstracts or other forms of grey literature compared to Embase, which could result in missed early-stage research or emerging trends.
2. Scope:
PubMed’s focus is largely on the clinical and biomedical fields, meaning that it may not always capture multidisciplinary studies or broader life science topics.
Embase: A Comprehensive European Complement
Embase, produced by Elsevier, is another leading biomedical and pharmacological database with over 32 million records. It covers all of the journals included in MEDLINE (and, therefore, PubMed) but extends beyond them by including an additional 3,000 journals, many of which are European and non-English.
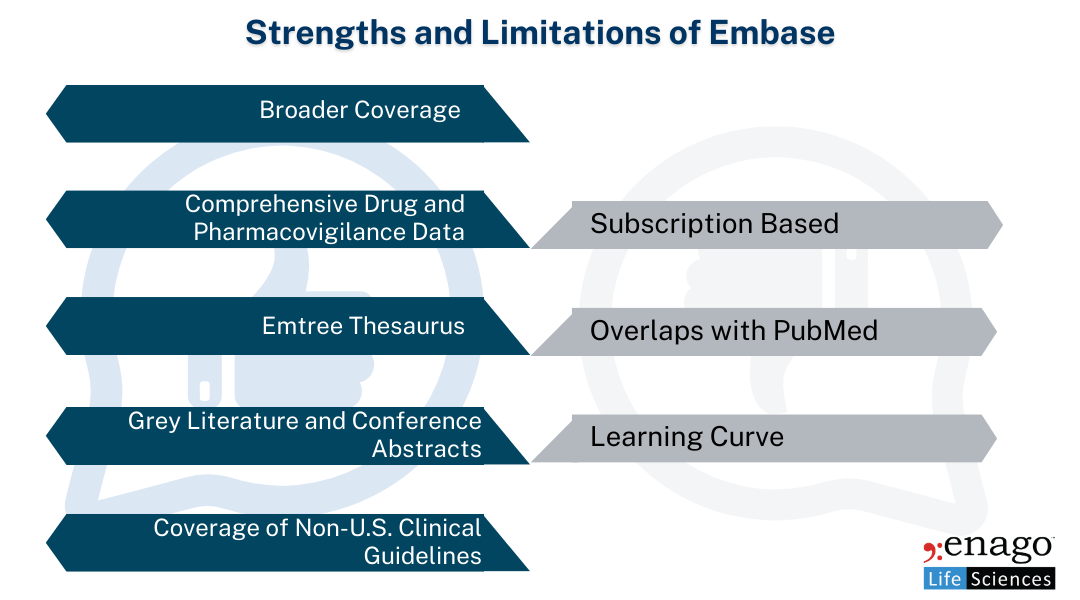
Strengths of Embase:
1. Broader Coverage:
Embase covers a more extensive range of journals, including European, non-English, and grey literature sources like conference abstracts, patents, and drug information. For medical communications professionals working on global projects, especially those targeting EMA (European Medicines Agency) guidelines, this is an invaluable resource.
2. Comprehensive Drug and Pharmacovigilance Data:
Embase excels in its indexing of pharmacology and drug research. It’s an essential database for medical communications writers who focus on pharmaceutical products, drug safety narratives, and post-marketing surveillance data.
3. Emtree Thesaurus:
Similar to PubMed’s MeSH terms, Embase uses the Emtree thesaurus to ensure precise indexing. Emtree is especially useful in pharmacovigilance and drug-related searches as it includes specific drug names, mechanisms, and pathways, offering deeper insights into pharmacology literature.
4. Grey Literature and Conference Abstracts:
Embase’s inclusion of conference proceedings and grey literature is a considerable advantage for medical communications, as it provides access to the latest, often unpublished research findings. This helps keep content cutting-edge, especially in fast-moving therapeutic areas.
5. Coverage of Non-U.S. Clinical Guidelines:
Embase often provides more comprehensive coverage of European and international clinical guidelines, which are critical when developing global regulatory submissions or international clinical trial documents.
Limitations of Embase:
1. Subscription-Based:
Unlike PubMed, Embase requires a paid subscription, which may limit accessibility for some users, particularly smaller medical writing or communication teams and medical practitioners.
2. Overlaps with PubMed:
While Embase includes all MEDLINE records, the significant overlap can sometimes lead to redundancy in literature reviews if not managed carefully.
3. Learning Curve:
While Emtree is a powerful tool, it can be more complex than PubMed’s MeSH terms, requiring more time and experience to use effectively, particularly for medical writers who are newer to the platform.
Practical Considerations in Choosing Between PubMed and Embase
From a Medical communications perspective, the decision of whether to use PubMed, Embase, or both often depends on the specific requirements of the project:
1. Geographical Focus:
For projects with a North American focus, PubMed alone may be sufficient. However, if the research spans European or global audiences, adding Embase to the mix will ensure more comprehensive coverage of international literature.
2. Regulatory Submissions:
When preparing documents for global regulatory submissions (e.g., both FDA and EMA), leveraging both PubMed and Embase ensures no stone is left unturned, especially for pharmacovigilance and drug safety documents.
3. Time Sensitivity:
Embase’s inclusion of grey literature and conference abstracts can help identify emerging trends and new research sooner than PubMed. This is especially valuable when time-sensitive or groundbreaking data are needed for competitive intelligence or rapid responses in communications.
PubMed and Embase in Combination
In many cases, using both databases offers the most thorough approach. A combined search strategy is particularly beneficial for systematic reviews, where comprehensive coverage of the literature is paramount. By cross-referencing results, medical writers can ensure that key studies are not missed, and that their analysis remains current, accurate, and global in scope.
Conclusion: The Right Tool for the Right Job
In medical communications, high-quality content development depends on high-quality research. PubMed and Embase each offer unique strengths and, in many cases, complement each other. Understanding their differences and knowing when to use each—or both—can significantly enhance the efficiency and quality of your literature reviews and analyses. Whether you are conducting a targeted search for U.S.-based clinical trials or developing a global pharmacovigilance report, these two databases are essential tools in the Medical communications arsenal.
By leveraging the strengths of both PubMed and Embase, medical communications professionals can ensure their work is grounded in comprehensive, accurate, and up-to-date scientific literature, thereby supporting the delivery of trusted, impactful content.
Further Reading:
- National Center for Biotechnology Information (NCBI) – PubMed Overview
https://pubmed.ncbi.nlm.nih.gov/ - Elsevier – Embase Overview
https://www.elsevier.com/solutions/embase-biomedical-research - S. National Library of Medicine – MeSH (Medical Subject Headings)
https://www.nlm.nih.gov/mesh/ - Elsevier – Emtree Thesaurus
https://service.elsevier.com/app/answers/detail/a_id/15174/supporthub/embase/ - Systematic Reviews in Health Care: Meta-Analysis in Context, 2nd edition – by Egger, M., Smith, G. D., Altman, D. G.
This text offers guidance on performing systematic reviews and the role of databases like PubMed and Embase. - Cochrane Handbook for Systematic Reviews of Interventions – by Higgins JPT, Thomas J, et al.
Available from Cochrane.org
Author:
 Dr. Anupama Kapadia
Dr. Anupama Kapadia
General Manager, Enago Life Sciences
Connect with Anupama on LinkedIn

