For decades, the world of medical publications was a predictable one. A clinical trial was completed, a manuscript was written, and it was submitted to a high-impact journal. The job of Medical Affairs was, in large part, to ensure this information was accurately and ethically disseminated.
In 2025, a milestone year, that model has become officially obsolete.
Medical Affairs (MA) has decisively shifted from a support function to the strategic “third pillar” of the biopharma industry, standing alongside R&D and Commercial. Its “currency”—scientific publications—is undergoing a radical transformation driven by data, digital technology, and a powerful new stakeholder: the patient.
Based on insights from 2025 industry reports and emerging trends, the future of publications isn’t about publishing anymore. It’s about scientific engagement. Here’s what that looks like.
Trend 1: The Patient is Now at the Publication Table
For years, “patient-centricity” was a corporate buzzword. In 2025, it is a tangible, measurable, and mandatory component of publication strategy.
The “why” is simple: patients are more informed, more engaged, and demand to be partners in their healthcare. The “how” is where Medical Affairs is innovating.
- Plain Language Summaries (PLS): This is the most significant tactical shift. The Good Publication Practice (GPP) guidelines (and the industry’s adoption of them) have cemented PLS as a non-negotiable. A 2024 survey of publication professionals found that over 82% view PLS as “vital” for research accessibility. In 2025, a publication plan without a PLS strategy is considered incomplete.
- Patient-Reported Outcomes (PROs): According to the ZS Medical Affairs Outlook Report 2025, as a product moves through its lifecycle, the demand for information from healthcare professionals (HCPs) and payers becomes “increasingly patient-focused.” They don’t just want to know if a drug works; they want to see the PROs, quality of life data, and patient experience.
- Co-Creation: Looking ahead, leading MA teams are moving beyond simply informing patients to involving This includes co-creating PLS with patient advocacy groups to ensure readability and relevance and even incorporating patient authors on manuscripts to provide context for the data.
Trend 2: The Rise of “Whole-Life” Data (RWE & HEOR)
Phase 3 clinical trial data is no longer the only story to tell. In 2025, it’s just the first chapter. Payers, regulators, and HCPs need to know a product’s value and performance in the messy, diverse real world.
This is where publications focused on Real-World Evidence (RWE) and Health Economics and Outcomes Research (HEOR) have become paramount.
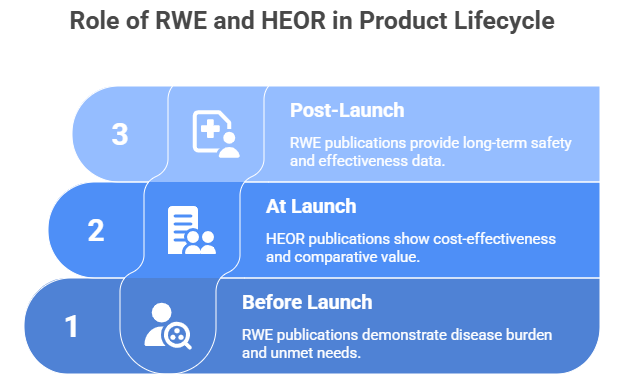
Reports from firms like McKinsey and IQVIA highlight that MA teams are now charged with “innovating evidence generation” across the entire product lifecycle.
- Before Launch: RWE publications are used to demonstrate the burden of a disease and identify unmet needs.
- At Launch: HEOR publications are critical for conversations with payers, demonstrating cost-effectiveness and comparative value.
- Post-Launch: RWE publications provide long-term safety and effectiveness data in diverse populations that were not included in the original trials.
A 2025 report from McKinsey notes that even regulators like the FDA are showing increased receptiveness to RWE, making these publications more critical than ever for demonstrating long-term value.
Trend 3: The Publication as the Start of the Conversation
Perhaps the biggest strategic shift is the “what’s next.” The journal article is no longer the final destination; it’s the fuel for a much broader, coordinated communication strategy.
This is the “Omnichannel” revolution. A key 2025 medical communications trend is the move toward integrated, omnichannel engagement.
Instead of an HCP accidentally stumbling upon a journal article, Medical Affairs now plans a meticulous, data-driven “scientific narrative” that leverages the core publication data across multiple channels:
- The “Anchor”: The peer-reviewed publication establishes scientific credibility.
- The “Engagers”: This core data is then repurposed into a consistent, personalized, and channel-appropriate format. This could include:
- An infographic for social media (targeting HCPs).
- A video abstract.
- A slide deck for a Medical Science Liaison (MSL) to use in a precision-targeted discussion.
- Content for a virtual advisory board.
- A podcast episode with the lead investigator.
The 2025-era MA team knows exactly which HCPs are seeing what information, ensuring a consistent scientific story and personalizing the data to that provider’s specific interests.
The “Engine” Driving It All: AI and Digital Transformation
None of these trends would be possible without the fourth, and most foundational, trend: AI-powered digital transformation.
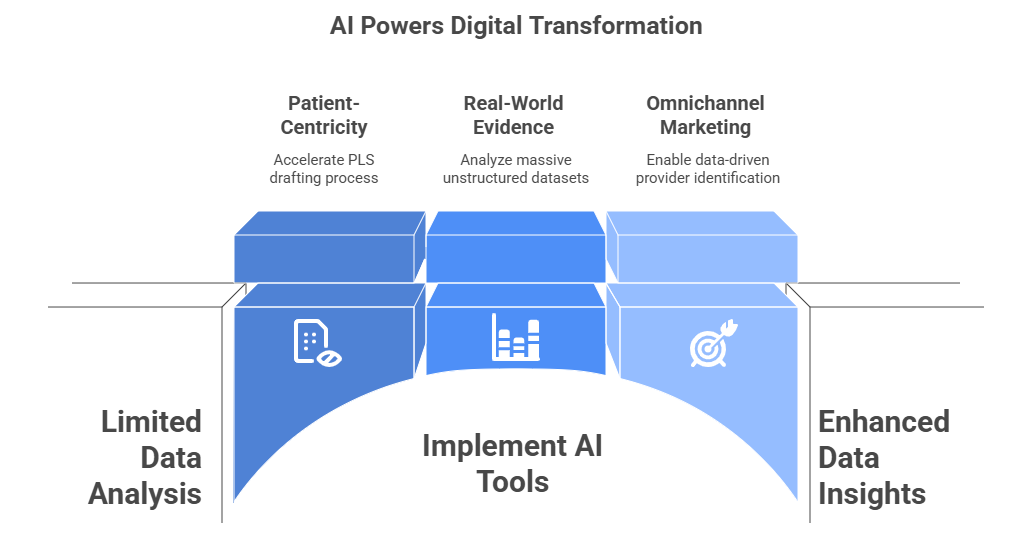
Artificial intelligence is the engine enabling this new, complex ecosystem.
- For Patient-Centricity: AI tools are being used to accelerate the drafting of PLS, ensuring they are at the correct reading level while maintaining scientific accuracy.
- For RWE: AI is the only way to analyze the massive, unstructured datasets (from electronic health records, insurance claims, and wearables) that constitute RWE, identifying patterns and unmet needs.
- For Omnichannel: AI platforms are what enable the “data-driven provider identification” that allows MA teams to move from broad-shot communication to precision targeting.
The Future: From Dissemination to Dynamic Engagement
The Medical Affairs and publications function of 2025 is proactive, data-driven, and deeply integrated.
The shift is clear:
- From Static (a PDF in a journal) to Dynamic (a living, multi-channel narrative).
- From Data Dissemination to Scientific Engagement.
- From Clinician-Focused to Patient-Partnered.
The future leaders in Medical Affairs will be those who master this new ecosystem—not just publishing data, but using it to build trust, demonstrate value, and, ultimately, improve outcomes for the patients they serve.
Successfully navigating this shift from static dissemination to dynamic, omnichannel engagement requires specialized expertise. As MA teams are tasked with integrating AI, disseminating complex HEOR/RWE data, and creating patient-centric content like Plain Language Summaries, many are turning to expert partners. Medical communications agencies like Enago Life Sciences specialize in providing this strategic support, from publication planning and medical writing to developing the digital and AI-driven solutions that define the 2025 vision.
Author:
 Dr. Anupama Kapadia
Dr. Anupama Kapadia
General Manager, Enago Life Sciences
Connect with Anupama on LinkedIn

