The integration of artificial intelligence (AI) into clinical trials is transforming how research is conducted, analyzed, and implemented. With the International Council for Harmonisation (ICH) E6(R3) guideline the future of clinical research stands at the intersection of technological innovation and robust ethical standards. This article explores how the ICH guideline can support the ethical and effective integration of AI into clinical trials, addressing opportunities, challenges, and best practices.
The Growing Role of AI in Clinical Trials
AI is reshaping clinical trials by enhancing efficiency, accuracy, and scalability. From patient recruitment to data analysis, AI offers numerous benefits, including:
- Enhanced Patient Recruitment: AI-powered tools analyze vast amounts of data to identify eligible patients, reducing recruitment timelines and improving trial diversity.
- Data Monitoring and Management: Machine learning algorithms monitor trial data in real time, flagging anomalies and ensuring data quality.
- Predictive Analytics: AI models predict trial outcomes, helping researchers design more effective trials and minimize risks.
- Personalized Medicine: AI facilitates adaptive trial designs by tailoring treatments to individual patient responses.
While these advancements promise to revolutionize clinical research, they also present challenges that require careful consideration.
Challenges of Integrating AI in Clinical Trials
Despite its potential, integrating AI into clinical trials is not without hurdles. Key challenges include:
- Data Privacy and Security: AI systems often require access to sensitive patient data, raising concerns about confidentiality and compliance with regulations like General Data Protection Regulation (GDPR) and Health Insurance Portability and Accountability Act (HIPAA).
- Algorithm Transparency: Black-box AI models can be difficult to interpret, making it challenging for researchers and regulators to validate their outputs.
- Bias in AI Models: AI algorithms trained on non-representative datasets may produce biased results, undermining the validity and generalizability of trial findings.
- Regulatory Uncertainty: The evolving nature of AI technologies poses challenges for regulatory frameworks. Clear and standardized guidelines on AI validation and approval are essential for widespread adoption.
How E6(R3) Supports AI Integration?
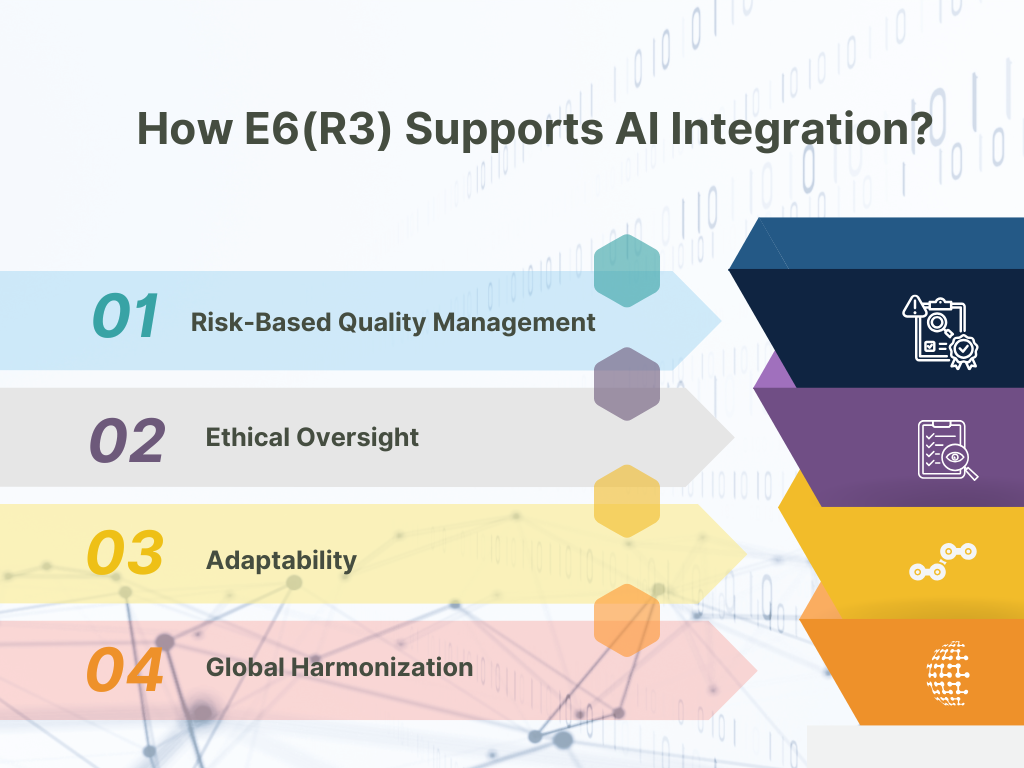
The ICH E6(R3) guideline emphasizes flexibility and risk-based approaches, making it a valuable framework for incorporating AI into clinical trials. Key principles that align with AI integration include:
- Risk-Based Quality Management: The guideline’s focus on identifying and managing risks proactively aligns with the need to address potential biases and errors in AI algorithms. AI developers and trial sponsors must validate AI tools to ensure they produce reliable and unbiased results.
- Ethical Oversight: Participant safety and data integrity are central to E6(R3). AI systems used in clinical trials must comply with ethical standards, including transparency, data privacy, and informed consent.
- Adaptability: E6(R3) encourages adaptability in trial designs, supporting the use of AI to optimize trial protocols and analyze complex datasets. For instance, adaptive trials enabled by AI can adjust parameters in real-time based on interim results.
- Global Harmonization: By fostering harmonization across regulatory bodies, E6(R3) facilitates the global adoption of AI-driven methodologies. This ensures consistency in standards and practices, regardless of trial location.
Best Practices for Implementing AI in Clinical Trials
To address these challenges and align with the ICH E6(R3) guideline, stakeholders should adopt the following best practices:
- Comprehensive Validation: Ensure AI systems undergo rigorous testing and validation to confirm accuracy, reliability, and mitigate bias.
- Transparent Algorithms: Use explainable AI models to enhance transparency and facilitate regulatory approval. Providing clear documentation of algorithm functionality is critical.
- Data Security Measures: Implement robust data encryption and anonymization techniques to protect patient information and ensure compliance with regulations.
- Interdisciplinary Collaboration: Engage experts from diverse fields, including data science, clinical research, and ethics, to develop and monitor AI-driven trials.
- Continuous Monitoring: Establish mechanisms for ongoing monitoring of AI tools during trials to detect and address potential issues promptly.
Case Studies: AI in Action
Several successful implementations of AI in clinical trials demonstrate its potential:
- Patient Recruitment:
Recruiting eligible participants for clinical trials is often time-consuming and inefficient. In a large-scale oncology trial, AI was used to analyze electronic health records (EHRs) to identify suitable candidates more quickly. This approach reduced recruitment time significantly compared to traditional methods, ensuring faster trial initiation and broader patient access (PMC10682526).
- Adaptive Trials:
Traditional clinical trials follow fixed protocols, which may not always be optimal for patient safety and treatment efficacy. An AI-driven adaptive trial for a rare genetic disorder utilized machine learning models to optimize dosing strategies dynamically. This resulted in improved patient outcomes by minimizing adverse effects and personalizing treatment based on real-time data (PMC10651639).
- Real-Time Monitoring:
Ensuring patient adherence in clinical trials is critical for data integrity, yet manual tracking is prone to errors. AI-powered monitoring tools were employed in a study involving diabetes patients, enabling real-time adherence tracking through wearable devices and predictive analytics. This led to enhanced data quality and better patient engagement (PMC8521858).
These examples highlight the transformative impact of AI while underscoring the importance of ethical and regulatory frameworks like ICH E6(R3).
The Road Ahead
As AI continues to evolve, its role in clinical trials will only expand. The ICH E6(R3) guideline provides a solid foundation for integrating these technologies while maintaining high standards of ethics and data integrity. However, collaboration between regulators, sponsors, and technology developers is essential to address emerging challenges and unlock AI’s full potential.
By embracing innovation and adhering to robust guidelines, the clinical research community can harness AI to accelerate medical advancements and improve patient outcomes. The future of clinical trials lies in this delicate balance of technology and humanity—a vision that the ICH E6(R3) guideline helps bring to life.
References:
- Askin, Scott, Denis Burkhalter, Gilda Calado, and Samar El Dakrouni. “Artificial intelligence applied to clinical trials: opportunities and challenges.” Health and technology13, no. 2 (2023): 203-213.
- Babel, Aditi, Richi Taneja, Franco Mondello Malvestiti, Alessandro Monaco, and Shaantanu Donde. “Artificial intelligence solutions to increase medication adherence in patients with non-communicable diseases.” Frontiers in Digital Health3 (2021): 669869.
- Chopra, Hitesh, Dong K. Shin, Kavita Munjal, Kuldeep Dhama, and Talha B. Emran. “Revolutionizing clinical trials: the role of AI in accelerating medical breakthroughs.” International Journal of Surgery109, no. 12 (2023): 4211-4220.
- Nashwan, Abdulqadir J., and Salam Bani Hani. “Transforming cancer clinical trials: The integral role of artificial intelligence in electronic health records for efficient patient recruitment.” Contemporary Clinical Trials Communications36 (2023): 101223.
- Wojtara, Magda, Emaan Rana, Taibia Rahman, Palak Khanna, and Heshwin Singh. “Artificial intelligence in rare disease diagnosis and treatment.” Clinical and Translational Science16, no. 11 (2023): 2106-2111
Author:

Rebecca D’souza, PhD.
Associate Content Expert, Enago Academy
Connect with Rebecca on LinkedIn

